Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron. Which of the following best describes the function of the nucleolus.

Which Substance Is The Oxidizing Agent In This Reaction 2cuo C 2cu Co2 In 2022 Oxidizing Agent Substances Agents
Chlorine Cl is the oxidizing agent because it gains an electron.

. Al is the oxidizing agent because its oxidation number decreases. Which best describes the oxidizing agent in this reaction. Contains the majority of the cells genetic material e.
The oxidizing agent causes oxidation of the reducing agent generating the loss of electrons of the substance and therefore oxidizes in the process. Whereas oxidation state of Br is changing from -2 to -3 this means Br is gaining electrons therefore it is an oxidizing agent. D A substance that reacts.
The oxidizing agent typically takes these electrons for itself thus gaining electrons and being reduced. An oxidizing agent is a specie which accepts electrons and gets reduced in a chemical reaction. The oxidizing agent is usually in one of its higher possible oxidation states as it will gain electrons and be reduced.
In the given reaction Oxidation state of Al changes from 0 to 3 therefore it is reducing agent. It is also known as an oxidizing agent to that element or compound that passes electronegative atoms to another substance. Which statement best identifies and describes the oxidizing agent in the reaction.
Which best describes the oxidizing agent in this reaction. Cl2aq 2Braq 2Claq Br2aq Bromine Br is the oxidizing agent because it gains an electron. More than one of the above is true.
Br2 is the oxidizing agent because its. It is also known as electron acceptor. 2al 3br2 2albr3 al is the oxidizing agent because its oxidation number increases.
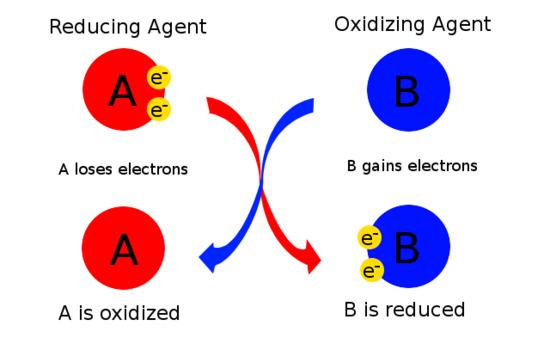
Bromine losses an electron and therefore it is the reducing agent while chlorine gains electrons and therefore it is an oxidizing agent. Heres a typical table of standard reduction potentials. An illustration detailing the electron-accepting properties of oxidizing agents is.
Oxidizing agents are also known as oxidants or oxidizers. The oxidizing agent typically takes these electrons for itself thus gaining electrons and being reduced. A oxidizing agent is a chemical substance that has the ability to subtract electrons from another substance reducing agent that donates or loses them.
The strongest oxidizing agent in the list is F2 followed by H2O2 and so on down to the weakest oxidizing agent Li. Formation of ribosomal subunits and ribosomal RNA b. As per this definition oxidizing agents are the reactants that undergo reduction in redox reactions.
As an electron acceptor They are chemical substances whose atoms remove at least one electron from another atom in a chemical reaction. An oxidizing agent oxidant gains electrons and is reduced in a chemical reaction. Cl2aq 2Braq 2Claq Br2aq Chlorine Cl is the oxidizing agent because it gains an electron.
When studying chemical reactions all the substances involved and the processes that occur in them. Chlorine Cl is the oxidizing agent because it gains an electron. Which best describes the oxidizing agent in this reaction.
The substance that is oxidized because it loses electrons b. Correct answer to the question Which describes the oxidizing agent in a chemical reaction. Which best identifies why the rusting of an iron nail in the presence of water and oxygen is an oxidation-reduction reaction.
Oxidizing agents can be defined in two different ways. The reducing agent undergoes oxidation loss of electrons in a chemical reaction. Nuclear protein synthesis c.
An oxidizing agent is thus an electron acceptor. An oxidizing agent is a reactant that removes electrons from other reactants during a redox reaction. Transfer of genetic information to the endoplasmic reticulum d.
1 on a question. 3 on a question Which describes the oxidizing agent in a chemical reaction. An oxidizing element or oxidizing agent is one that reaches a stable energy state as a result of which the oxidant is reduced and gains electrons.
Br2 is the oxidizing agent because its oxidation number increases. Bromine Br is the oxidizing agent because it loses an electron. Chlorine Cl is the oxidizing agent because it gains an electron.

Oxidizing Agent How To Discuss
0 Comments